Scientists Develop Artificial Chemical Receptor to Assist Viral Transduction for T Cell Engineering
Engineered T cell immunotherapy, such as chimeric antigen receptor T cell (CAR-T) and T cell receptor T cell (TCR-T) therapy, has emerged as a potent therapeutic strategy for treating tumor.
However, the genetic manipulation for primary T cells remains inefficient, especially during the clinical manufacturing process. There’s an urgent need to develop a reliable method for the preparation of engineered T cells.
A research team led by Prof. CAI Lintao at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, in collaboration with Prof. MA Yifan from the HRYZ (Shenzhen) Biotechnology co. LTD, developed a "safe, efficient and universal" technique based on bioorthogonal chemistry and glycol-metabolic labeling for viral-mediated engineered T cell manufacturing.
In this strategy, the functional azide motifs were anchored on T cell surface via the intrinsic glycometabolism of exogenous azide-glucose, serving as an artificial ligand for viral binding. The complementary functional moiety dibenzocyclooctyne (DBCO)/-conjugated PEI1.8K (PEI-DBCO) was coated on lentiviral surface, which strengthened the virus-T cell interaction through DBCO/azide bioorthogonal chemistry.
"We found that this artificial chemical receptor effectively facilitated viral binding to T cells and elevates the transduction efficiency of the lentivirus from 20% to 80% without any effect on T cell proliferation and activity” Prof. CAI said, “This artificial chemical modification was also appropriate for introducing other heterologous gene into T cells, including GPF, CAR and TCR, indicating a great potency for universal T cell engineering”.
The technique has been demonstrated to be safe for human primary T cells as well, without interference of the cell expansion or anti-tumor functions. When put into the CAR-T preparation, PEI-DBCO/azide-glucose system significantly increased the yield of CAR T cells and boosted their anti-tumor effect both in vitro and in the B lymphoma xenograft mouse model with a low dose CAR-T cells, which will reduce clinical adverse effects.
"This artificial chemical labeling strategy is an effective, safe and easy upgrade for viral-based gene manipulation of human primary T cells, thereby showing a great potential for clinical engineered T lymphocytes manufacture, including CAR-T and TCR-T cell therapy,” said Prof. CAI. “I hope that this brand-new technology we have developed here will achieve the manufacturing of engineered T cells to clinical application and industrialization as soon as possible."
The paper "Glycometabolic Bioorthogonal Chemistry-Guided Viral Transduction for Robust Human T Cell Engineering" was published in Advanced Functional Materials.
Prof. CAI, the corresponding author of this paper, was selected as Fellow of American Institute for Medical and Biological Engineering (AIMBE) on 25th March at American Academy of Sciences in Washington, which is a non-profit organization and was founded in 1991, for his remarkable contribution in optical probes and biomimetic drug delivery systems in the fields of nanomedicine and cancer theranostics.
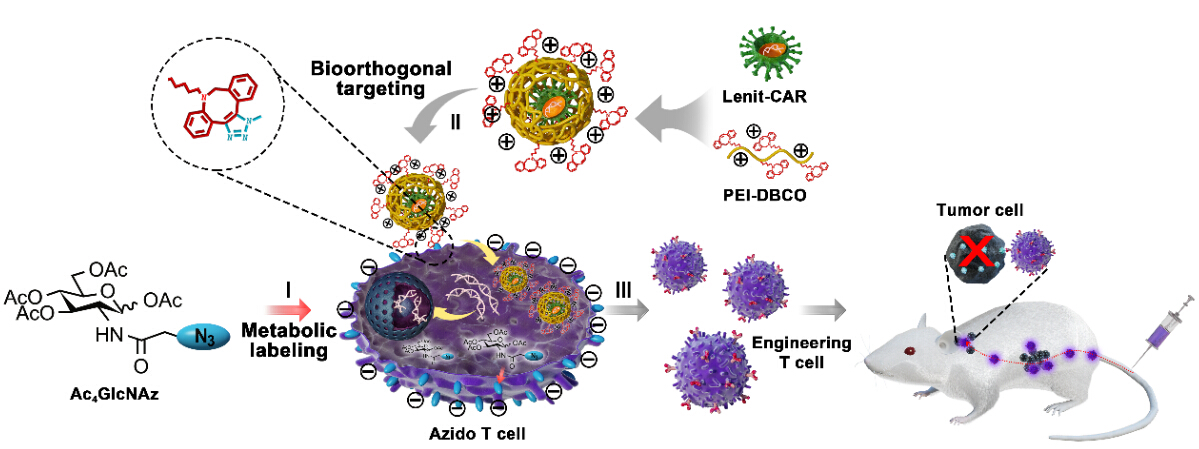
Fig. Application of artificial chemical receptor in CAR-engineered T cell manufacturing and immunotherapy of tumor. (Image by Prof.CAI)

AIMBE Fellow Certification
CONTACT:
ZHANG Xiaomin
Email: xm.zhang@siat.ac.cn
Tel: 86-755-86585299
| Download the attachment: |
|
|